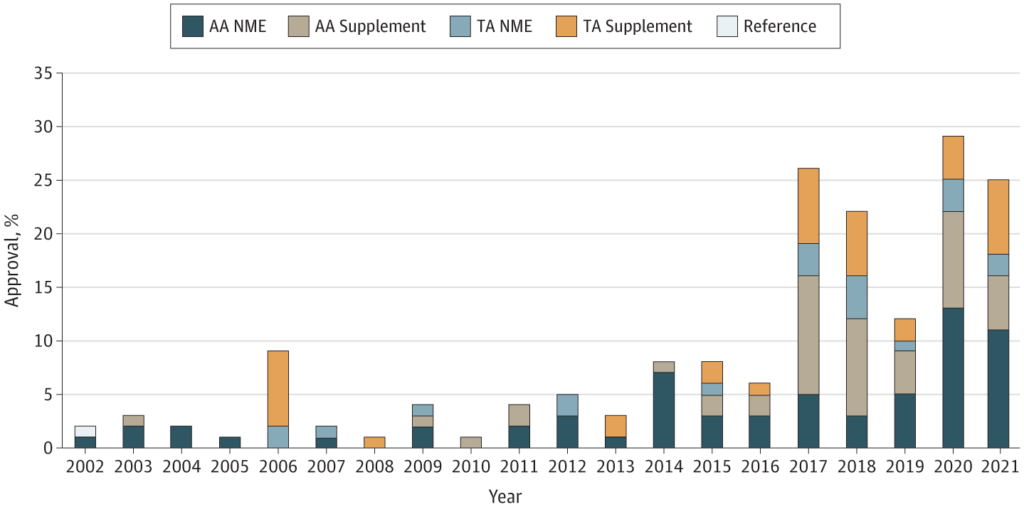
These have gotten more and more widespread. Agrawal et al. (2022) use knowledge between 2002-2021, and located that FDA permitted:
…176 new malignant hematology and oncology indications based mostly on single-arm trials, together with 116 accelerated approvals (AAs) and 60 conventional approvals. Total, 87 approvals (49%) have been for brand new molecular entities or unique biologics and 89 (51%) have been supplemental indication. Response fee (RR) was the commonest finish level used to assist approval in these single-arm trials (173 of 176 [98%]). Of the 116 AAs based mostly on single-arm trials, 45 (38%) fulfilled their postmarketing requirement to confirm scientific profit, 61 (52%) are pending verification of profit, and 10 (9%) have been withdrawn from the market as of December 31, 2021.